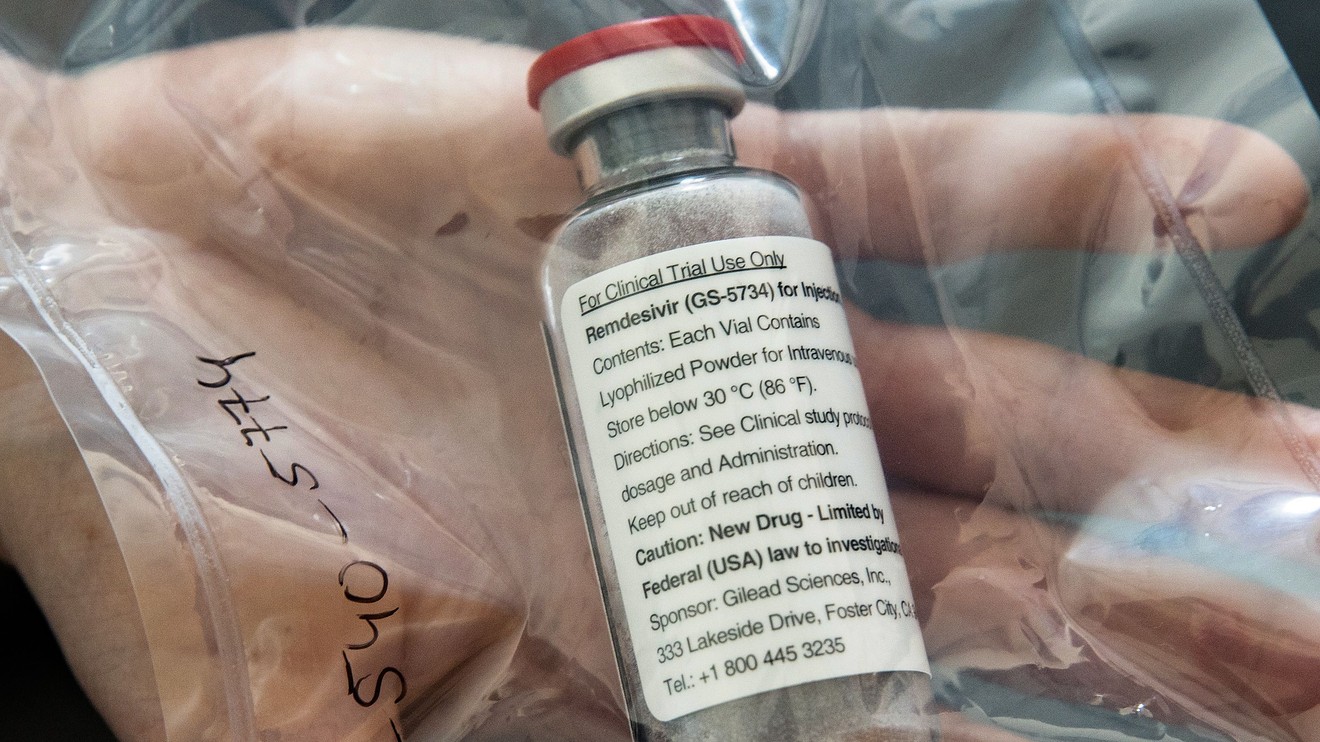
Analysts say early data showing Gilead Sciences Inc.’s remdesivir improved clinical outcomes for two-thirds of a small group of severely ill COVID-19 patients is promising, but they caution against viewing the experimental drug as a silver bullet in the fight against the novel coronavirus.
Shares of Gilead GILD, +2.40% were up 1.3% in trading on Monday; the stock has gained 15.3% since the start of the year.
The data showed that 68% of 53 hospitalized patients showed clinical improvement after taking the drug. It’s a “promising first look,” J.P. Morgan’s Cory Kasimov wrote in a note on Monday. “The results need to be kept in context. It’s difficult, if not impossible, to make any firm conclusions from an uncontrolled data set with a small sample size.”
See also:These 21 companies are working on coronavirus treatments or vaccines — here’s where things stand
The findings, which were published Friday in the New England Journal of Medicine, examined the use of remdesivir, a drug that Gilead has also been testing as an Ebola Virus Disease treatment, in COVID-19 patients who have been hospitalized. These patients received the drug through Gilead’s compassionate use program, not through a formal clinical trial. (As of April 4, more than 1,700 people have been treated with remdesivir through Gilead’s compassionate and expanded use programs, CEO Daniel O’Day has said.)
Patients in nine countries each received their first dose of remdesivir around March 7; about 68% showed clinical improvement, 47% were discharged, and 13% died, and no new safety issues were raised in this group of patients. The analysis didn’t have a preset number of patients, site locations, or duration.
The mortality data, in particular, was flagged by some analysts as not really noteworthy.
“Our conclusion is, unfortunately, remdesivir may not be doing much,” Raymond James analyst Steven Seedhouse told investors on Monday. “We hope we’re wrong.”
“We believe remdesivir could show benefit and clinical improvement; however, we cannot draw definitive conclusions from a compassionate use data given the limitations (such as small sample size, lack of controls and randomization and short follow-up periods),” SunTrust Humphrey Robinson analysts wrote April 10.
That’s an issue that Gilead’s chief executive acknowledges. O’Day, who has taken to sharing updates about remedesivir every weekend for the past three weeks, said in a letter published on Friday: “In studying remdesivir, the question is not just whether it is safe and effective against COVID-19 but in which patients it shows activity, how long should they receive treatment and at what stage of their disease would treatment be most beneficial.”
There are no proven treatments or vaccines for COVID-19, the infection caused by the virus that was first identified in Wuhan, China, late last year and has since sickened nearly two million people and killed more than 100,000 people. In the U.S., some hospital systems are stretched to the limit, health care workers themselves have been sickened or even died from the virus, and in some cases two patients are being hooked up to the same ventilator. The Food and Drug Administration has used its emergency powers to grant a number of emergency use authorizations to therapies like hydroxychoroquine and blood purification devices given the lack of available treatment options.
There’s a lot of hope riding on remdesivir, which is being studied in multiple trials, in the U.S., and abroad, including two conducted by Chinese health authorities. It has also been touted by President Donald Trump. However, the investigational therapy has never been approved by regulators in any country for any reason.
Read:Gilead Sciences, ‘flooded’ by demand for remdesivir, halts compassionate use
There are at least 11 clinical trials under way, in the U.S. and abroad, evaluating remdesivir in COVID-19 patients, according to ClinicalTrials.gov, including an adaptive, randomized, double-blind, placebo-controlled trial with 440 participants that is being run by the National Institute of Allergy and Infectious Diseases. Trial data is expected to be published this month, including from two trials in China. (O’Day said in his note that a Chinese study of patients with severe COVID-19 had been halted due to “stalled enrollment” and publication of that data will come from Chinese authorities.)
Gilead is expected to share findings from two Phase 3 trials evaluating remdesivir in moderate and severe COVID-19 patients in May. Last week the drugmaker increased the number of participants in those trials, from 600 to 1,600 patients in the moderate trial, and from 400 to 2,400 in the severe trial. It also updated the trials’ endpoints to include a measure of improvement in hospital outcomes, according to SunTrust analysts.
Year-to-date, the Health Care Select Sector SPDR Fund XLV, -0.87% has dropped 6.9%, while the S&P 500 SPX, -1.01% is down 13.6%.










Add Comment