The first U.S. authorization of a COVID-19 vaccine on Friday provided a ray of hope in a pandemic that has killed more than 300,000 Americans, but limited vaccine supply means initial doses will go to the highest-priority groups — and the average American will likely have to sit tight for a few months.
A vaccine candidate developed by Germany’s BioNTech BNTX, +2.71% and commercialized by Pfizer PFE, -1.28% started arriving at hospitals nationwide Monday, following its emergency-use authorization by the Food and Drug Administration three days earlier.
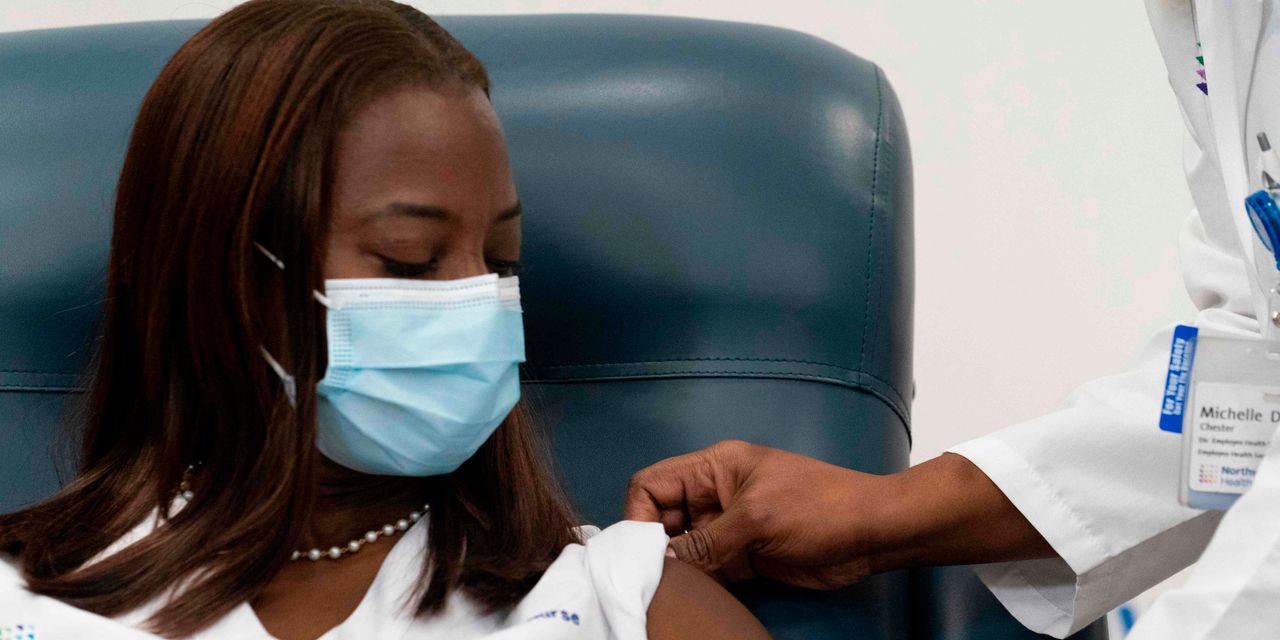
One of the country’s first vaccine doses to be administered outside of a clinical trial went to Sandra Lindsay, a critical-care nurse in Queens, N.Y., which was devastated by COVID-19 cases and deaths in the pandemic’s early months.
“I hope this marks the beginning to the end of a very painful time in our history,” Lindsay said after receiving her shot during a livestreamed news conference with New York Gov. Andrew Cuomo. “I want to instill public confidence that the vaccine is safe — we’re in a pandemic, and so we all need to do our part to put an end to the pandemic and to not give up so soon.”
Her inoculation was in line with the Centers for Disease Control and Prevention’s recent recommendation that health-care workers and long-term-care facility residents — two populations at particularly high risk for contracting COVID-19 — be first in line to receive vaccine doses. The CDC’s Dec. 3 guidance was based on a recommendation from the independent Advisory Committee on Immunization Practices (ACIP) panel.
“The recommendations were made with these goals in mind: decrease death and serious disease as much as possible; preserve functioning of society; [and] reduce the extra burden COVID-19 is having on people already facing disparities,” the CDC notes.
Essential workers, people with chronic health conditions that put them at greater risk for severe COVID-19, and adults 65 and older are also expected to be prioritized for vaccine doses going forward.
But for the average American, “the earliest will likely be in the spring, but more likely over the summer,” Sandra Albrecht, a Columbia University assistant professor of epidemiology and chief epidemiologist for the science-communication project Dear Pandemic, previously told MarketWatch.
“ ‘The goal is for everyone to be able to easily get a COVID-19 vaccination as soon as large quantities of vaccine are available. As vaccine supply increases but remains limited, ACIP will expand the groups recommended for vaccination.’ ”
Lt. Gen. Paul Ostrowski, the director of supply, production and distribution for Operation Warp Speed, the government’s vaccine development and distribution effort, said in an MSNBC interview last month that by June, “100% of Americans that want the vaccine will have had the vaccine.”
“The goal is for everyone to be able to easily get a COVID-19 vaccination as soon as large quantities of vaccine are available,” the CDC says. “As vaccine supply increases but remains limited, ACIP will expand the groups recommended for vaccination.”
The Pfizer-BioNTech candidate, which requires that recipients return for a second shot three weeks after their initial dose, showed 95% efficacy in a late-stage clinical trial. It was granted emergency-use authorization, a less-rigorous clearance than full FDA approval that is being used to accelerate the use of coronavirus treatments and vaccines.
There still aren’t enough data to determine the Pfizer vaccine’s safety in people who are immunocompromised, children younger than 16 and people who are pregnant or lactating, according to an FDA briefing document.
A vaccine candidate from Moderna Inc. MRNA, -5.06%, which has shown 94.5% efficacy in protecting against COVID-19, could also receive authorization after an advisory-committee meeting scheduled for Thursday. Ahead of that meeting, the FDA said Tuesday that Moderna’s vaccine candidate is “highly effective” at preventing coronavirus infections.
Experts say people will still need to wear masks and practice social distancing for some period of time even after they’re vaccinated, since it remains to be seen whether the vaccines will prevent asymptomatic infection and how long immunity will last.
For more answers to your vaccine questions, check out MarketWatch’s guide here.










Add Comment