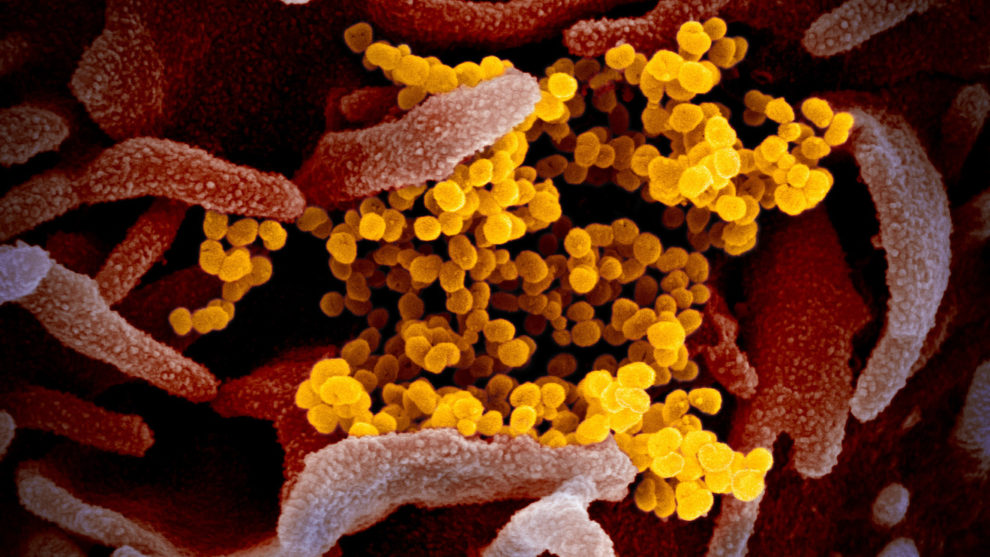
Shares of Moderna Inc. MRNA, +27.81% soared 28% in trading on Tuesday as the biotechnology company took the lead in efforts to develop a vaccine for COVID-19, the novel coronavirus that has sickened more than 80,000 people and killed 2,600 people worldwide.
Moderna said Tuesday that it had shipped the first batch of its experimental vaccine to the National Institute of Allergy and Infectious Diseases (NIAID), setting up mRNA-1273 to be first vaccine to be tested in the U.S. in humans in a Phase 1 clinical trial.
A mix of small biotechs and legacy drugmakers, including GlaxoSmithKline Plc GSK, -1.69% , Johnson & Johnson JNJ, -0.86% , Moderna and Sanofi SNY, -1.67% , are working on vaccine development in partnership with governments around the world. Gilead Sciences Inc. GILD, -3.84% , J&J and Regeneron Pharmaceuticals Inc. REGN, +3.99% have also announced plans to test their drugs as potential treatments for COVID-19 infections. (J&J’s technology is being used to screen therapeutic candidates.)
The COVID-19 outbreak, which originated in December in Wuhan, China, this week roiled markets as the number of infections jumped in countries outside of Asia, particularly in Iran and Italy.
There are no proven therapies that treat COVID-19 or vaccines that prevent infection. However, a number of companies have individually announced plans to develop therapies, and others are partnering with government agencies and public health organizations like Biomedical Advanced Research and Development Authority (BARDA), which is part of the Department of Health and Human Services, and the Coalition for Epidemic Preparedness Innovations (CEPI), a public-private vaccines organization that was founded in 2017 at the World Economic Forum.
CEPI, for example, paid to manufacture the first batch of Moderna’s vaccine candidate. During a call with reporters on Tuesday, Dr. Anthony Fauci, NIAID’s director, said Moderna’s vaccine candidate is “on time and maybe even a little bit better.” The vaccine, however, still needs to go through Phase 2 and 3 clinical trials to test for efficacy and response rate. It will take at least a year to a year-and-a-half for a vaccine to complete the regulatory process, Fauci said.
Gilead’s stock was down 4% on Tuesday. The drugmaker is conducting a clinical trial in China for its investigational antiviral remdesivir as a treatment for COVID-19 infections. The University of Nebraska Medical Center in Omaha, Neb., which is treating some of the repatriated passengers from the Diamond Princess cruise ship in Yokohama, Japan, said in a public filing this week that it is conducting a clinical trial testing remdesivir as a treatment for COVID-19 infections.
Getting a treatment for COVID-19 would be a “game-changer,” Fauci said.
Moderna’s stock has gained 18% over the past three months, while the SPDR S&P Biotech XBI, -3.03% is up 6%.
div > iframe { width: 100% !important; min-width: 300px; max-width: 800px; } ]]>