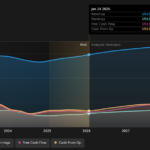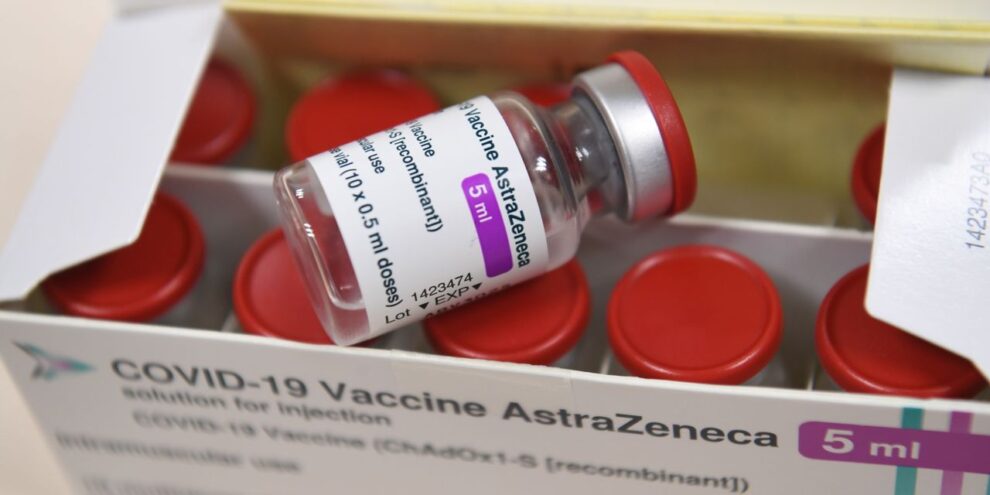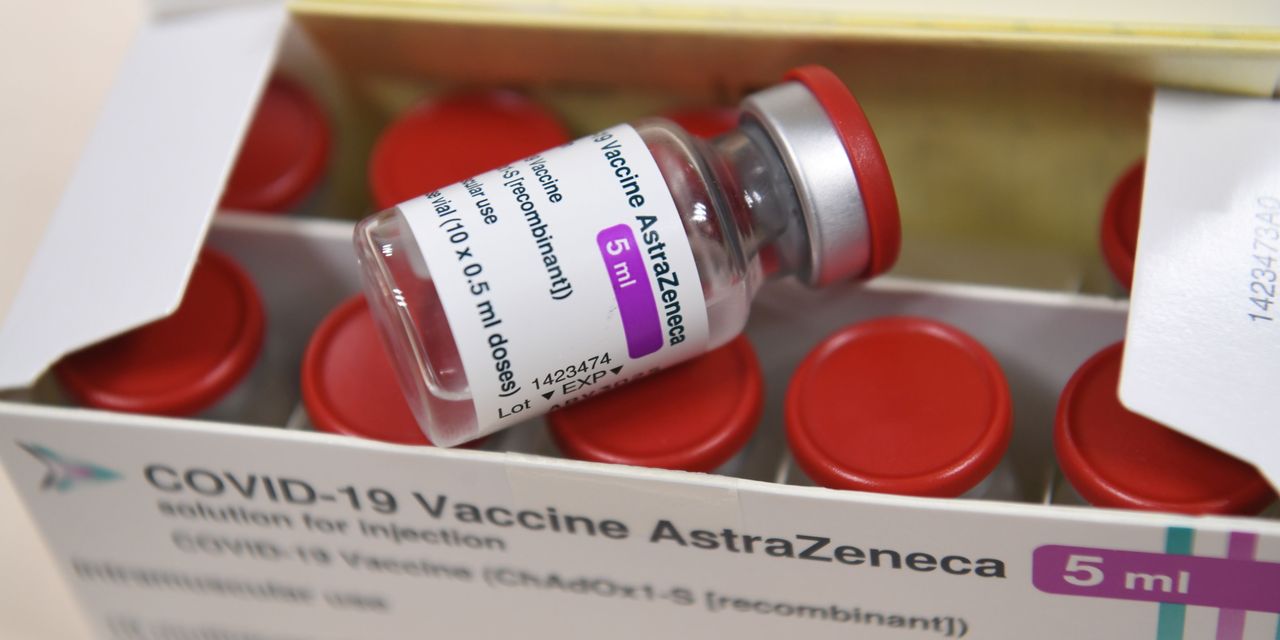
A single dose of either the AstraZeneca–Oxford vaccine or the Pfizer–BioNTech COVID-19 shot cuts the risk of hospital admission among older adults by more than 80% percent, Public Health England (PHE) said, citing a preprint study.
The real world study, which was published on Monday and hasn’t yet been peer-reviewed, showed that protection against any COVID-19 symptoms in those over 70 ranged between 57% and 61% for one dose of the Pfizer–BioNTech shot, and between 60% and 73% for the AstraZeneca–Oxford one four weeks after the first shot.
PHE said the data suggested the vaccine from U.S. drug company Pfizer PFE, +0.60% caused an 83% reduction in COVID-19 deaths among the over-80s. There were no equivalent data for the vaccine from Anglo-Swedish drug company AstraZeneca AZN, -0.60%, which began to be administered at a later date.
Read: Pfizer vaccine can reduce transmission after 1 dose, new study finds
“This adds to growing evidence showing that the vaccines are working to reduce infections and save lives,” said Dr. Mary Ramsay, PHE head of immunization.
“While there remains much more data to follow, this is encouraging and we are increasingly confident that vaccines are making a real difference,” she added.
Both Pfizer’s German partner BioNTech BNTX, +1.08% and AstraZeneca AZN, +0.07% are looking into the efficacy of vaccines against new strains of the coronavirus that causes COVID-19, such as those identified in South Africa and Brazil. Last month, AstraZeneca said it could take between six and nine months to produce COVID-19 vaccines that are effective against new variants.
Read: Pfizer–BioNTech COVID-19 vaccine likely to protect against South Africa strain
U.K. health minister Matt Hancock described the results of the study as “very strong,” and said that they “may also help to explain why the number of COVID admissions to intensive-care units among people over 80 in the U.K. have dropped to single figures in the last couple of weeks.”
More than 20 million people, or more than 30% of the U.K. population, have now received their first dose of a COVID-19 vaccine, with the elderly and those most at risk at the top of the priority list.
Almost 816,000 people have had their second dose, according to the latest government data.
Read: France won’t give AstraZeneca coronavirus vaccine to those over 65
The new data should also help allay concerns in some European countries, including France, Germany and Italy, which recommended that the vaccine developed by AstraZeneca with the University of Oxford should not be used for people over 65, citing insufficient data on its efficacy for older people.
On Tuesday, the French government revised its stance, saying that older people, including those aged between 65 and 74, with pre-existing conditions, could receive the AstraZeneca–Oxford vaccine from primary-care surgeries, hospitals and “within days” from pharmacies.
Read: As new infections keep rising in France, Macron resists lockdowns
Those aged over 75 in France will still be offered either the Pfizer–BioNTech vaccine or the shot made by U.S. biotech Moderna MRNA, +1.67%, in a vaccination center, said French health minister Olivier Véran, speaking on television.
Germany’s vaccine commission is also reviewing its recommendation. On Sunday, Prof. Carsten Watzl, secretary-general of the German Society for Immunology, urged the country to start allowing the over-65s to receive the AstraZeneca–Oxford vaccine.
“We do know that the vaccine works in that age group. The recent data from Scotland clearly show it elicits an immune response, the elderly are protected from severe disease by this vaccine,” said Watzl in a BBC interview.
Preliminary findings from a study released last week, which was conducted by the Universities of Edinburgh and Strathclyde and Public Health Scotland, and covered the entire Scottish population, showed that by the fourth week after the initial dose, the Pfizer–BioNTech and AstraZeneca–Oxford vaccines were found to reduce the risk of hospitalization by up to 85% and 94% respectively.